Common Uses of Tannic Acid Powder
Tannic acid, a type of polyphenolic compound made up of gallic acid molecules and glucose, is found in tea, nettle, wood, and berries. It exhibits astringent, constricting properties, antioxidant, and antimicrobial activity, among others, and has a broad range of applications across various industries. It has been used for centuries as a natural medicine for the treatment for diaper rash, cold sores, and fever blisters. It also helps improve wound healing and reduce postoperative hemorrhage. In this overview, we will discuss the chemical composition, structure, and properties of tannic acid along with its common uses.
What’s Tannic acid powder?
Tannic acid, also known as gallotannin, is a polyphenolic compound belonging to the family of hydrolysable tannins. Tannic acid is naturally found in a variety of plant sources, such as oak bark, sumac leaves, and the insect gall nuts of Pistacia lentiscus. Extraction methods usually involve soaking the plant material in water, alcohol, or a combination of both. Tannic acid powder is a yellowish-white that readily dissolves in water, alcohol, and acetone. It has a bitter taste with astringent properties due to the presence of multiple phenolic hydroxyl groups that can bind and precipitate proteins. This characteristic makes it valuable in various applications, including leather tanning and dyeing.
Tannic acid’s antioxidant nature arises from its ability to scavenge free radicals, while its antimicrobial activity results from inhibiting bacterial and fungal cell wall synthesis. Furthermore, it has metal-chelating properties, enabling it to interact with metal ions, making it useful for water treatment and corrosion protection.
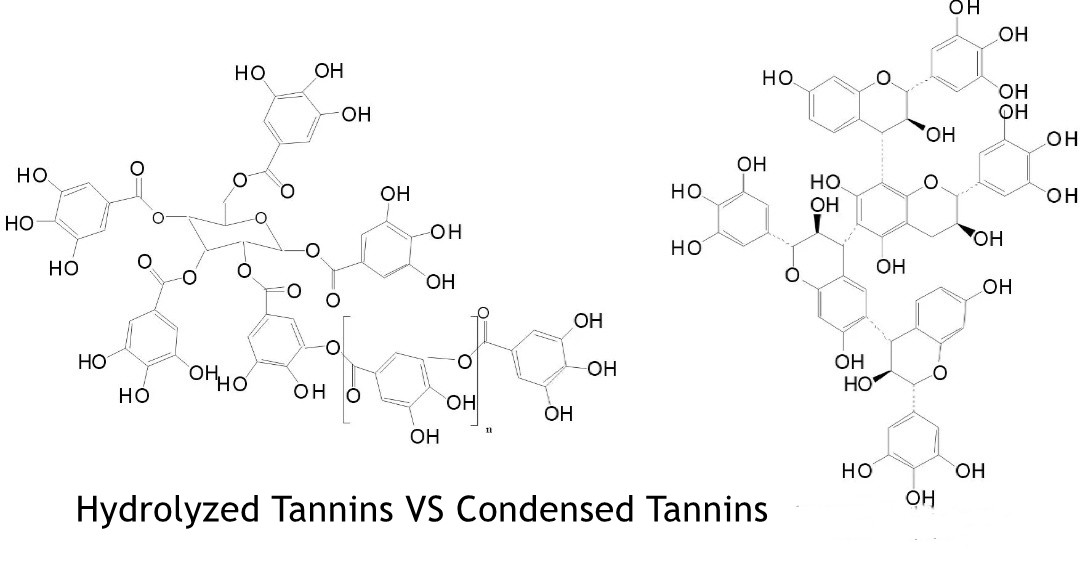
Chemical Composition and Structure
Its molecular formula is C₇₆H₅₂O₄₆, and its structure consists of multiple gallic acid units linked together by ester bonds. The central core contains a glucose molecule esterified with multiple gallic acid units, and the free hydroxyl groups of these gallic acid units may be further esterified with additional gallic acid molecules. The overall structure is highly complex and variable, resulting in a heterogeneous mixture of different tannins and polysaccharides.
Common uses of Tannic acid
Numerous industries utilize tannic acid due to its versatile properties:
- Leather industry: Tanning involves treating raw animal hides, stabilizing the collagen fibers, and preventing decay. Tannic acid aids in this process, creating supple and durable leather.
- Textile industry: Tannic acid serves as a natural dye and mordant, bonding colorants to fabric fibers to produce a long-lasting color.
- Food industry: Its antioxidant properties make tannic acid valuable as a preservative and flavor enhancer, especially in the production of wine and tea.
- Medical applications: Tannic acid’s astringent properties are beneficial for treating wounds or alleviating inflammation.
- Water treatment: Tannic acid acts as a chelating agent, removing metal ions from water sources.
- Natural dye: Ink Making The main component of ink is tannic acid powder and a ferrous sulfate solution. These ingredients can be purchased commercially and are easily extracted from their source materials. A tannic acid powder that is ground and mixed with iron sulfate will produce a water-soluble tannate complex. This tannate complex will then change to ferric tannate, a darker pigment that is difficult to erase. Ink made with tannic acid is similar to traditional European gallic ink and can be produced from local raw materials. Wood Staining Staining wood is a great way to highlight the natural grain and color of the wood. This will create a wash of tannic acid onto the wood that you can then sand again for an even finish.