The Difference Between Steroidal and Triterpenoid Saponins
Saponins are a diverse group of natural ingredients found in plants. The water solution of this compound can produce a large number of persistent, soap-like foam after shaking, so-called saponins. It can be divided into steroidal saponins and triterpenoid saponins according to their different chemical structures. Generally, saponins consist of non-polar triterpene or sterol backbones and hydrophilic sugar moieties. They have a wide range of biological activities, including plant defense against herbivores and disease. Many saponins have been shown to exhibit a wide range of pharmacological activities including hypocholesterolemic, anti-coagulant, anticarcinogenic, hepatoprotective, hypoglycemic, immunomodulatory, neuroprotective, anti-inflammatory and anti-oxidant activity. Today here we will introduce the difference between steroidal and triterpenoid saponins.
What are Triterpenoid Saponins and Steroidal Saponins?
Triterpenes are widely distributed in nature. Some triterpenes exist in plants, which are called Triterpenoid sapogenins. Some combine with sugar to form glycosides, called Triterpenoid saponins. A terpene compound consisting of 30 carbon atoms with 6 isoprene units in the molecule, C5H8 6. Triterpenoid saponins have carboxyl group, so they are also called acidic saponins, mostly exist in the form of free or glycosylated ester.
The structure of saponins can be divided into glycosides and sugars. If the aglycones are spirosteroids, they are called steroidal saponins, which are oligosaccharides derived from spirosteroids. There are many kinds of sugar in steroid saponins, which is significantly different from triterpene saponins. So far, more than 10 species have been discovered, including hexose, pentose, deoxyse, ketose, uronic acid, etc. Among them, D-glucose, D-galactose, D-xylose, L-rhamnose and L-arabinose are the most common.
Structure
Triterpene saponins are composed of six isoprene units. According to the number of 30 carbon atoms in aglycones, they can be divided into tetracyclic triterpenes (lanolin type, damarane type, glycerane type, cycloartenane, cucurabane, meladiane and pentacyclic triterpenes) and pentacyclic triterpenes (oleanane, ursulane,lupane, xylocane).
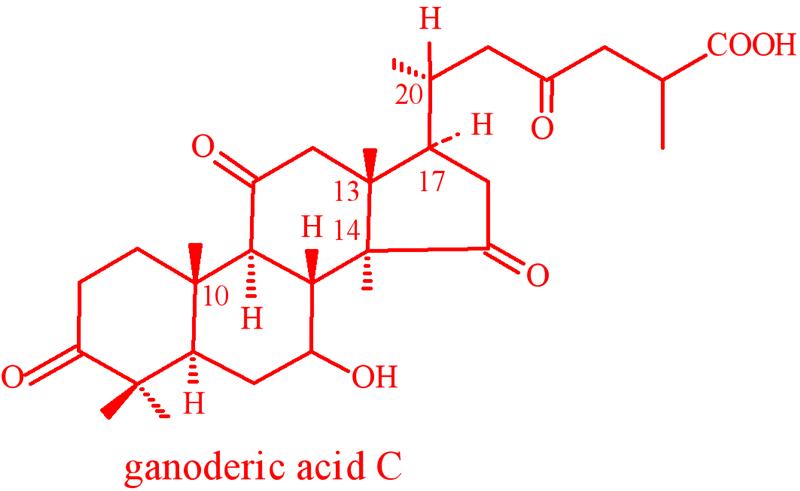
A typical Triterpene saponins
The structure of steroidal saponins is determined by the aglycone skeleton and the sugar moiety. The aglycone skeleton is composed of 27 carbon atoms, which are divided into three subclasses: spirostan, furostan and cholestane saponins. In addition to the aglycone skeleton, each steroid molecule has one or more sugar moieties, and each sugar is assigned a specific position in the steroid skeletal structure, which is defined by the number of carbon atoms that the sugar occupy in the parent steroid molecule. Steroid saponins are glycosides containing one or more oligosaccharide chains attached to a steroidal aglycone with specific skeletal features and localised substituents. The aglycone is usually positioned at the 3-OH position, and the biological activity of saponins depends on both their aglycone and sugar moiety.
Source
Triterpenes (tritnes) are widely distributed in nature, including fungi, ferns, monocotyledonous plants and dicotyledonous plants. They are mainly distributed in caryophyllaceae, Aracanthaceae, leguminous plants, coniferous trees, polygonaceae, Platycodontidae and scrophulariaceae. Herbs containing triterpenoid saponins include ginseng, licorice, bupleurum, astragalus, platycodon, neem bark, ganoderma lucidum, etc. Steroidal saponins are a group of secondary metabolites, originating from a variety of different plant families, including the Fabaceae, Araliaceae, Caryophyllaceae, Liliaceae and Dioscoreaceae families. These components are mainly distributed in monocotyledonous plants, most of which exist in Liliaceae, Dioscoreaceae, Lycoraceae and agave family. A series of steroidal saponins with special structures have been isolated from various Marine organisms and animals.
Use
Triterpenoid saponins have been studied intensively in recent years. Ginsenosides can promote RNA protein biosynthesis, regulate body metabolism and enhance immune function. Saikosaponins inhibit the central nervous system, have significant anti-inflammatory effects, and reduce plasma cholesterol and triglyceride levels. Aesculin has obvious anti-exudation, anti-inflammatory and anti-stasis effects, can restore the normal permeability of capillaries, improve capillary tension, control inflammation, improve circulation, and has a better therapeutic effect on brain trauma and cardiovascular diseases.
Steroidal saponins are natural metabolites which possess diverse biological activities. Several of these saponins have been shown to exhibit significant and immunity enhancer and antitumor activity. For example, The main components of the yucca schidigera extract were steroidal saponins (Sarsapogein, Smilagenin, Smilagenin, Hecogenin), Saponin — free, saponin and Glyco — components. Due to its special physiological structure, it has a strong adsorption capacity for harmful gases. It can be used in animal breeding to reduce the concentration of harmful gases such as ammonia and hydrogen sulfide, improve the breeding environment of livestock and poultry, and enhance the body immunity. It can also stimulate the circulatory and respiratory system, affect vitamin activity, animal hormone secretion, and has the function of pancreatic emulsifier. Tigogenin 3-O-b-d-Glc-(1 – 2)-[b-d-Glc-(1-3)]-b-d-Glc-(1-4)-b-d-Gal stimulated apoptosis in colorectal cancer SW480 cells by increasing caspase activation, reducing Bcl-2 family proteins expression and generating ROS production (Wang et al. 2013c). Their antitumor activity is characterized by their capacity to induce programmed cell death in different tumor cells lines. They can modulate oncogenic processes (cancer cells proliferation, migration, apoptosis), induce angiogenesis and sensitize cancer cells to chemotherapy. Cytotoxicity of steroidal saponins has been studied for many years and most of the experimental data were performed in vitro on cancer cell lines.
How to distinguish Triterpenoid Saponins and Steroidal Saponins
The foam produced after the saponin solution is related to the PH of the solution. The neutral saponins produce stable foam under alkaline condition, but the foam is not stable under acidic condition. Steroidal saponins/acid saponins produce bubbles with the same persistence under alkaline or acidic conditions, so several chemical reactions can be used to distinguish steroidal saponins from triterpenoid saponins
- Acetic anhydride-concentrated sulfuric acid reaction (Liebermann-burchard)
The sample is dissolved in acetic acid, and concentrated sulfuric acid-acetic anhydride (1:20) is added to produce red → purple → blue → green → dark green and other changes until fading. Steroidal saponins also show this reaction but change more quickly and finally appear dark green; Triterpenoid saponins change slightly more slowly, and do not appear dark green, only red or purple.
- Trichloroacetic acid reaction (Rosen-Heimer reaction)
The sample solution was placed on filter paper and sprayed with 25% trichloroacetic acid ethanol solution. Steroidal saponins showed red to purple spots when heated at 60 ℃, while triterpenoid saponins showed color only when heated at 100℃.
- Antimony pentachloride reaction (kahlenberg reaction)
The sample alcohol solution was placed on the filter paper, sprayed with 20% antimony trichloride (or antimony pentachloride) chloroform solution (which should not contain ethanol and water) and dried, and heated at 60-70 ℃, which showed yellow, gray blue, gray purple spots, and blue-purple fluorescence under ultraviolet lamp (steroidal saponins showed yellow fluorescence).